 |
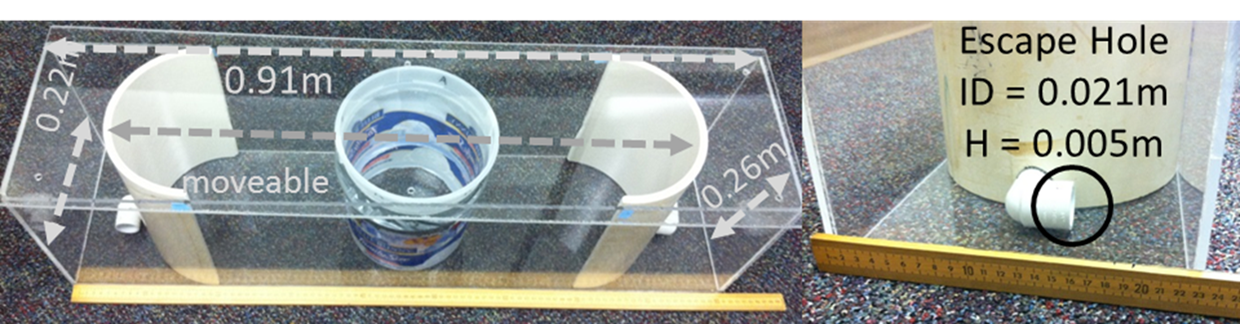
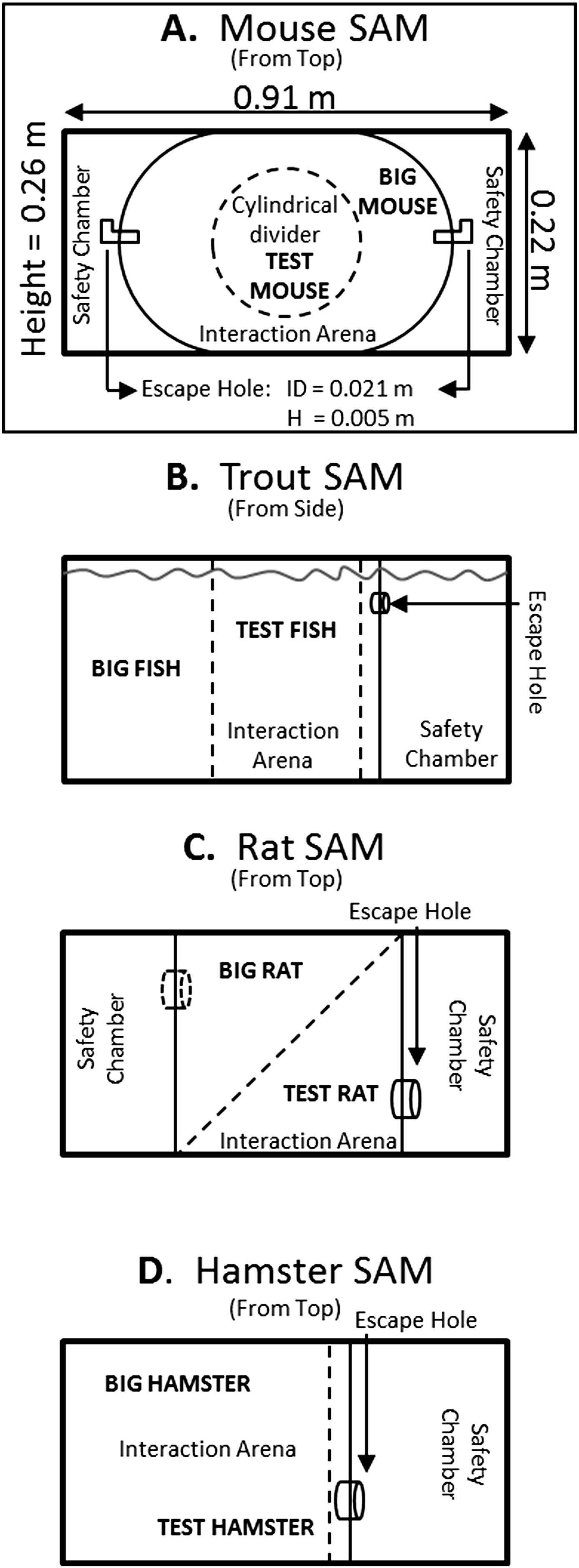
Figure 1. - Schematic depictions of developmental iterations of the Stress-Alternatives Model (SAM) designed for mice, trout, rats, and hamsters. A) The current SAM apparatus designed for rodents (dimensions are for the mouse version), including mice, rats, and hamsters. This version has no corners, an oval open field with flexible dimensions and two escape routes only large enough for the smaller test animal. A test rodent is placed inside the center circular opaque divider (dashed line); the divider is removed after presentation of a tone (conditioned stimulus, CS) to initiate trials. Trials may be social interactions with the addition of a larger conspecific placed outside the circular divider prior to the start. Arena and escape routes are novel for the initial trial, and L-shaped tunnels obscure the destination, creating some anxious behavior (delayed latency to escape) on their initial use. B) The SAM was first tested with rainbow trout in a 284 L aquarium where a test animal is fed. A removable opaque divider (dashed line) was used to separate the larger aggressor and intruder prior to interaction; its removal followed cessation of water flow (CS). A second removable opaque divider (dashed line) allows access to an escape hole large enough for only the smaller test fish, located near the top of the water column, which leads to the safety chamber. C) Initial experiments with rats utilized a square arena, and a single escape hole and safety chamber. A second escape hole and safety chamber were added to prevent the larger aggressive animal from blocking access to the safety chamber. A diagonal opaque divider (dashed line) separated the large rat from test rats, and was removed following a short tone (CS), prior to social interaction. D) Golden hamsters reliably escape social aggression, and this version of the SAM was designed to study the effect of forced interaction and submission versus escape. A removable divider (dashed line) could be used to block access to the escape hole and safety chamber for smaller individuals, forcing some hamsters to submit to the larger aggressor.
|
 |
Figure 6. - Familiarity reduces anxiety. A gradient of anxious behavior (open field ? social interaction ? social defeat) is verified by latency to escape and plasma corticosterone concentrations in mice (READ FROM THE BOTTOM UP). The data are normalized to novel escape latency (A, B), latency to escape after control vehicle drug treatment on day 3 (C), undisturbed control baseline corticosterone concentrations (D, E, F). LEFT COLUMN, LATENCY TO ESCAPE: A) Open Field (OF) test using SAM reveals significantly faster escape from the OF (left hatched clear bars) on repeated trials (familiarity), and even faster escapes after anxiolytic NPS treatment (double hatched gray bars;*significantly [P < 0.05] different from novel escape latency = 0%; #significantly reduced from OF only). B) Faster escape occurs with repeated trials (familiarity) during social aggression (Agg Esc; clear hatch), and even faster escapes after anxiolytic running wheel treatment (gray double hatch; *significantly different from novel escape latency during social interaction = 0%; #significantly reduced from Agg Esc). C) Anxiogenic treatment (yohimbine = a2 antag; clear hatch) increases latency to escape in previously escaping animals, while anxiolytic treatment (antalarmin = CRF1 antag; gray double hatch) reduces latency in previously submissive animals (*compared to vehicle baseline, +compared to anxiolytic, #compared to anxiogenic). RIGHT COLUMN, PLASMA CORTICOSTERONE CONCENTRATIONS: D) Familiarity with escape (clear hatch) results in low corticosterone secretion (not different from undisturbed controls, and the addition of the anxiolytic NPS (gray double hatch) significantly reduces corticosterone concentrations (*compared to undisturbed controls). E) Potent glucocorticoid response after repeated exposures to aggression and escape (Agg Esc; clear hatch), is reduced by exposure to a running wheel (gray double hatch; *compared to controls; #compared to Agg Esc alone). F) The largest increase in corticosterone is found in submissive animals following social interaction (Agg Sub; clear hatch), which is reduced by escape (compare to 5E clear hatch), or access to running wheel (gray double hatch; *compared to control concentrations, #significantly reduced compared to Agg Sub).
|